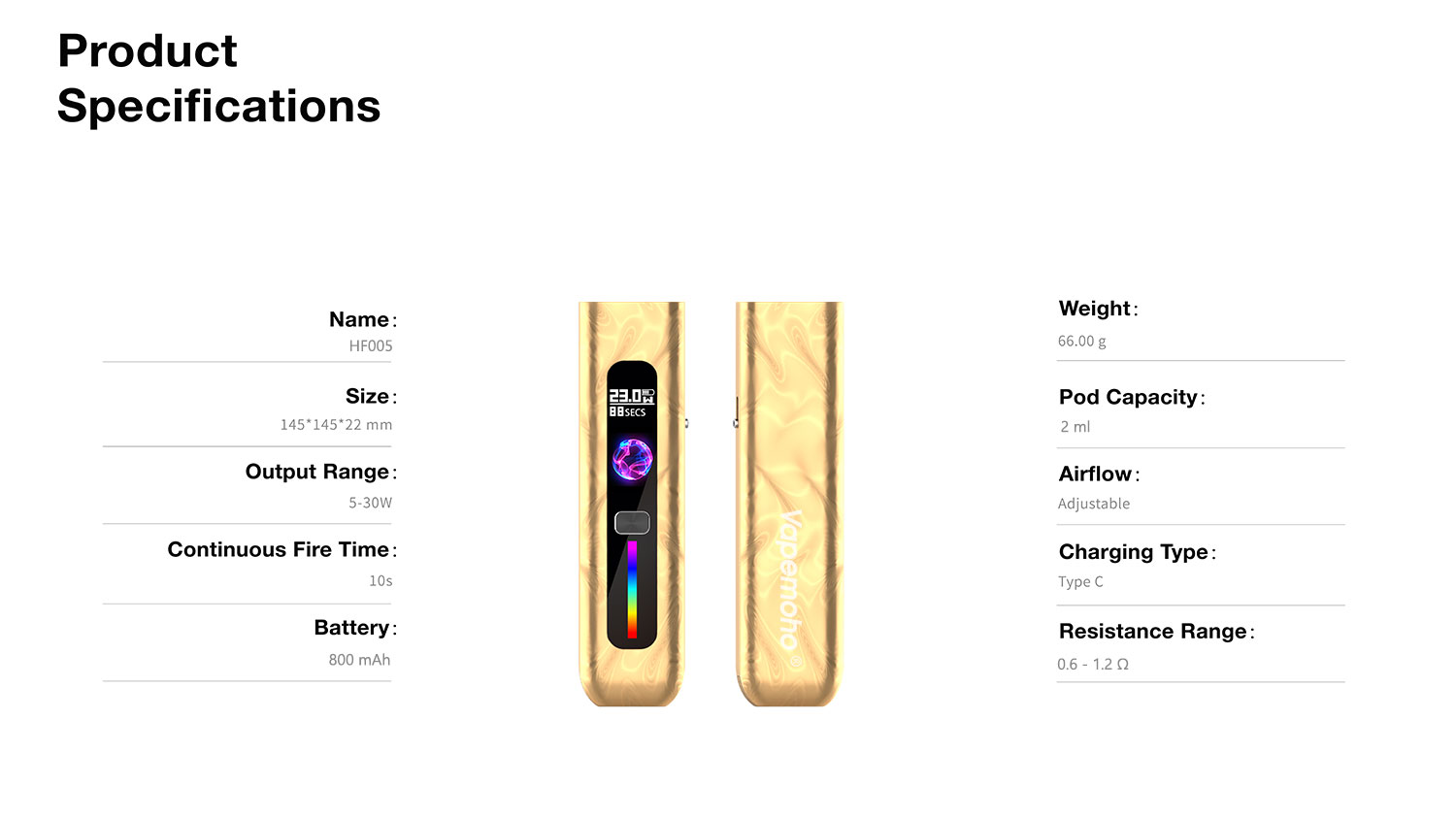
The evolution of e-cigarette manufacturing has gone through significant changes, moving from traditional production methods to more advanced automated systems. With the growing demand for e-cigarettes worldwide, factories must adopt intelligent automation to stay competitive. This shift not only increases production efficiency but also ensures higher quality, safety, and consistency in the final product. In this article, we explore how e-cigarette factories are upgrading their production processes with cutting-edge technologies.
1. The Rise of Intelligent Automation in E-Cigarette Factories
Intelligent automation is revolutionizing e-cigarette factories, providing a major boost to production speed and accuracy. Traditional factories relied heavily on manual labor, with workers assembling each component by hand. However, the increased demand for e-cigarettes and the need for high-quality standards have made automation essential. Automated systems can now handle everything from assembly and testing to packaging, all while maintaining consistency and reducing human error.

2. How Automation Enhances Production Efficiency
One of the key benefits of automation is its ability to significantly increase production efficiency. Robots and automated machinery can work tirelessly without breaks, which results in higher output and faster production cycles. This is particularly important for meeting the global demand for e-cigarettes. Automation also helps reduce labor costs, as fewer human workers are needed on the factory floor, allowing factories to allocate resources more effectively to other areas like research and development.
Moreover, automation allows for more precise control over the manufacturing process. Each e-cigarette component, such as the atomizer, battery, and cartridge, must be made with high precision to ensure safety and functionality. Automated machines can carry out complex tasks like soldering, testing, and assembling with a level of accuracy that is difficult to achieve manually. This results in fewer defects and a more consistent product.
3. Quality Control: Automation and Consistency
Maintaining high-quality standards is crucial for the success of e-cigarette factories. The use of intelligent automation in quality control processes ensures that each product meets rigorous safety and quality standards. Automated systems can perform real-time inspections, checking for issues like incorrect assembly, faulty batteries, or leaks in the liquid cartridges. These systems can also measure vapor consistency, ensuring that each device delivers a satisfying experience for the user.
By integrating AI and machine learning into the production process, factories can further improve their quality control. AI systems can identify patterns in production data and detect potential defects before they become widespread problems. This predictive capability enables factories to make adjustments in real-time, preventing large-scale recalls and ensuring a high-quality product is consistently delivered to consumers.
4. Adapting to Global Demand and Market Expectations
As the e-cigarette market continues to expand globally, factories must be agile enough to adapt to changing consumer demands and market expectations. Intelligent automation allows factories to scale production quickly, ensuring that they can meet rising demand without compromising quality. Furthermore, automation enables factories to produce a wider range of products, including different e-cigarette models, flavors, and nicotine concentrations, all while maintaining consistent quality.
This flexibility is particularly important in an industry where trends can change rapidly. Automation systems can be easily reprogrammed or adjusted to accommodate new product designs or changes in production requirements. This responsiveness helps factories stay ahead of competitors and ensures that they can quickly respond to shifts in consumer preferences.
5. The Future of E-Cigarette Manufacturing: Intelligent Factories
The future of e-cigarette manufacturing is undoubtedly tied to continued advancements in intelligent automation. As factories integrate more advanced technologies, such as artificial intelligence, robotics, and IoT (Internet of Things), the potential for innovation in the industry will only grow. These technologies will enable even greater efficiency, higher quality, and more personalized products for consumers.
In addition, smart factories will be able to monitor their operations in real-time, allowing manufacturers to identify inefficiencies, reduce waste, and make data-driven decisions. This shift towards intelligent manufacturing not only benefits the factory but also contributes to sustainability by minimizing energy usage and waste production. The future of e-cigarette factories will be defined by their ability to seamlessly integrate automation, AI, and other smart technologies to create a more efficient, flexible, and sustainable production process.
Conclusion
The move from traditional manufacturing to intelligent automation in e-cigarette factories is not just a trend but a necessity for staying competitive in an increasingly globalized market. Automation enhances production efficiency, ensures consistent quality, and provides the flexibility needed to meet changing market demands. As the industry continues to grow, the role of automation in e-cigarette factories will only become more crucial in shaping the future of production.