In the highly competitive electronic cigarette (e-cig) market, private mold design has become a critical strategy for brands to differentiate products, enhance user experience, and build brand loyalty. However, private mold development is often accompanied by challenges such as soaring costs, delayed timelines, and unforeseen risks. This guide breaks down actionable strategies to manage costs, optimize timelines, and mitigate key risks, helping e-cig brands navigate the private mold design process successfully.
Cost Management: Balance Quality and Affordability
Cost overruns are one of the most common pitfalls in e-cig private mold design. To avoid this, brands must adopt a proactive cost-control approach from the initial design phase—starting with material selection and mold structure optimization.

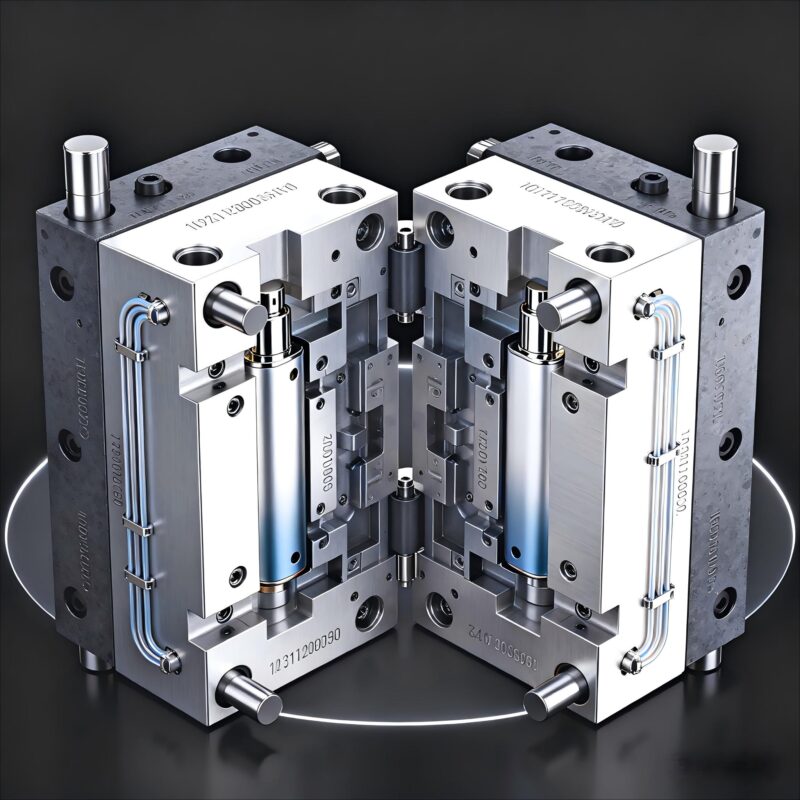
Figure 1: Common materials for e-cig private molds (left: FDA-approved ABS blends; right: mold steel components)
1.1 Material Selection: Prioritize Cost-Efficiency Without Compromising Compliance
E-cig molds require materials that meet food-grade and heat-resistant standards (e.g., FDA-approved plastics for mouthpieces). While high-end materials like medical-grade PC (polycarbonate) offer durability, they can increase mold costs by 15-20%. Instead, brands can opt for cost-effective alternatives such as ABS (acrylonitrile butadiene styrene) blends, which meet safety standards and reduce material costs by 8-12%. Additionally, working with suppliers to bulk-purchase raw materials can lower per-unit costs by 5-7%.
1.2 Mold Complexity: Optimize Design for Production Efficiency
Overly complex designs (e.g., intricate airflow channels, custom-shaped casings) increase mold manufacturing time and cost. For example, a mold with 4 cavities (for mass production) costs 30% more than a 2-cavity mold, but boosts production efficiency by 60%. Brands should balance design uniqueness with manufacturability: simplify non-critical features (e.g., minor surface patterns) and use standard mold components (e.g., prefabricated guide pins) to cut mold costs by 10-15%.
1.3 Supplier Negotiation: Build Long-Term Partnerships
Choosing the right mold supplier is pivotal for cost control. Avoid selecting suppliers solely based on low quotes—cheap molds may have poor precision, leading to costly reworks. Instead, partner with suppliers with e-cig industry experience and negotiate flexible terms: for instance, a 5% discount for long-term contracts (e.g., 2+ years) or shared tooling costs for future mold modifications. This approach can reduce overall mold costs by 8-10%.
2. Scheduling Timelines: Streamline the Development Process
Delayed mold delivery can cause missed market windows, especially in the fast-paced e-cig industry. A typical e-cig private mold project takes 8-12 weeks, but efficient timeline management can shorten this to 6-9 weeks—with clear phase division being the key.
Figure 2: Optimized timeline for e-cig private mold development (including 1-week buffer for contingencies)
2.1 Phase-by-Phase Timeline Planning
Break the project into clear phases with deadlines:
- Design Confirmation (2-3 weeks): Finalize 3D models (using CAD software) and conduct prototype testing (e.g., airflow performance, ergonomics). Involve engineers and marketing teams early to avoid last-minute design changes.
- Mold Manufacturing (4-6 weeks): Monitor supplier progress weekly—key steps include CNC machining (3-4 weeks) and mold testing (1-2 weeks). Use project management tools (e.g., Asana, Trello) to track milestones.
- Mass Production Preparation (1-2 weeks): Test mold output (e.g., check for burrs, dimensional accuracy) and debug production lines. Align with assembly teams to ensure seamless transition to mass production.
2.2 Buffer Time for Contingencies
Reserve 10-15% of the total timeline for unexpected issues (e.g., design reworks, material shortages). For example, if the mold testing phase fails due to airflow leaks, a 1-week buffer allows for adjustments without delaying the entire project. Without buffer time, 60% of e-cig mold projects experience delays of 2+ weeks.
3. Avoiding Key Risks: Proactive Mitigation Strategies
E-cig private mold design faces unique risks, from regulatory non-compliance to technical failures. Proactive risk management is essential to protect investments.
3.1 Regulatory Risks: Align with Market Standards
E-cig regulations vary by region (e.g., EU’s TPD, US FDA’s PMTA). A mold designed for the EU market may not comply with US size restrictions (e.g., minimum tank capacity), leading to mold obsolescence. To mitigate this, research target market regulations before design: for example, ensure mold dimensions meet FDA’s 2ml maximum e-liquid capacity rule. Conduct third-party compliance testing (e.g., RoHS for material safety) to avoid costly redesigns.
3.2 Technical Risks: Validate Designs Early
Design flaws (e.g., weak structural points, poor heat dissipation) can cause mold damage or product recalls. Use 3D printing to create prototypes and test for durability (e.g., drop tests, heat resistance). For example, a prototype with a faulty battery compartment can be revised before mold manufacturing, saving $5,000-$10,000 in rework costs. Additionally, conduct mold trial runs with small batches (50-100 units) to identify issues before mass production.
3.3 Supply Chain Risks: Diversify Suppliers
Overreliance on a single supplier can lead to delays if they face capacity issues (e.g., labor shortages, equipment breakdowns). Maintain 2-3 alternative suppliers for critical components (e.g., mold steel, plastic resins) and sign backup agreements. For example, if a primary supplier’s CNC machines fail, a backup supplier can take over within 3 days, minimizing timeline disruptions.
Conclusion
E-cig private mold design requires a balance of cost control, timeline efficiency, and risk mitigation. By optimizing material selection, streamlining project phases, and proactively addressing regulatory and technical risks, brands can develop high-quality private molds that meet market demands while protecting their bottom line. Remember: successful mold design is not just about creating a unique product—it’s about managing the entire process to deliver value efficiently.